
by Thomas Lethenborg | Aug 21, 2020 | News, Press releases
Quality Policy and Standards
As a technology leader in mobile health (mHealth) solutions for mental health, Monsenso is ISO 13485 certified. ISO 13485 is the gold standard for medical device companies to ensure the quality, safety and efficacy of products in the medical device field. This certification ensures that the product in question, consistently meets customer requirements and regulatory requirements applicable to medical devices and other related services.
“Monsenso adheres to the highest security standards. Beyond, being ISO 13485 certified, Monsenso holds the ISO 27001 certification and class 1 CE mark.” says Thomas Lethenborg, CEO at Monsenso.

For additional information contact:
Jennifer Highland
Marketing and Communications Manager
Monsenso
+45 81 71 7713
highland@monsenso.com

by Thomas Lethenborg | Jan 31, 2017 | News, Press releases
Copenhagen, Denmark – 31 January 2017. Monsenso ApS, Region Zealand, and two German partners have been granted major funding by Eureka/Eurostars, for the “IMProving Availability and Cost-effectiveness of mental Healthcare for Schizophrenia through mHealth” (IMPACHS) project.
The objective of the IMPACHS project is to design and develop a mobile and context-aware Cognitive Behaviour Therapy (CBT) content integrated into Monsenso Clinic. Through IMPACHS, Monsenso will deliver smartphone-based intervention technology for outpatients diagnosed with schizophrenia, to improve the availability and cost-effectiveness of mental health care.
Eurostars is the largest programme of knowledge-intensive SMEs with the objective of stimulating growth through international cooperation on the development of new products and services.
With regards to the grant, Thomas Lethenborg, CEO at Monsenso said “This grant will allow us to shift from being a remote patient monitoring digital tool to providing intelligent treatment to individuals with schizophrenia and other mental disorders over time. This will be the third project where we work on a similar solution since RADMIS and ENTER (previously called E-Mental) have similar goals, but for patients with bipolar disorder and borderline personality disorder respectively.”
The programme is a jointly funded initiative between the EU and 36 Eurostars countries in Europe and around the world, including Canada, South Korea, South Africa, Turkey, and Israel. During the first round of qualifications, there were 370 submissions, from which 61 projects were from Denmark. When the final qualification round took place, 19 Danish projects were selected to receive significant funding.
The Danish Innovations Fund is the local representative of Eureka/Eurostars in Denmark, and its total budget to distribute amongst all Danish projects is 43 million DKK.
For additional information contact:
Jennifer Highland
Marketing and Communications Manager
Monsenso
+45 81 75 80 01
highland@monsenso.com
To read the original press release in Danish issues by the Danish Innovations Fund, click here.

by Thomas Lethenborg | Dec 13, 2016 | News, Press releases
Copenhagen, Denmark – 13 December 2016. Monsenso, an innovation leader in mobile health (mHealth) technology for mental health, announced today that Psykiatriresurs, a private practice clinic in Gothenburg, Sweden, will implement the Monsenso mHealth solution for depression treatment.
The private clinic, which offers psychological and neuropsychiatric services, provides mental health treatment to 600 individuals with depression, anxiety, personality disorders and ADHD. In addition to these patients, Psykiatriresurs will soon open a second clinic with a similar capacity, in Varberg, 80 km South of Gothenburg.
To start with, the mHealth solution for depression will be used by 250 patients, although Dr. Kristoffer Södersten, Director of the clinic, has plans to roll it out to all patients across both clinics.
“With this new technology, we want to raise the bar on the mental health treatment offered around the different private clinics in Sweden, and with the Monsenso mHealth solution we will be able to go a step further by reaching out to our patients when they need it the most. At Psykiatriresurs we are constantly looking to improve our patients’ experience, and our aim is to use the technology to improve and personalise even more of the treatments we currently provide to our patients,” said Dr. Södersten.
The mHealth solution for depression will be used by the clinicians to support the personalised treatment of individuals with this disorder. With the web portal, they will be able to identify the triggers and early-warning signs of all patients and schedule emergency consultations when needed.
Thomas Lethenborg, CEO at Monsenso, said “With the implementation of the Monsenso mHealth solution for depression, clinicians at Psykiatriresurs will have access to real-time patient monitoring and their patients’ historical data, which will help them make better decisions in regards to clinical treatment.”
Patients will use a smartphone app to collect sensor data and fill in routine self-assessments that reveal their current state of mind. The smartphone app also works as a self-management tool that allows patients to manage their symptoms.
Clinicians will use a web portal where they can access all the data collected by the patients’ smartphones, anytime, anywhere. The web portal enables clinicians to view relevant information related to each patient.
For additional information contact:
Jennifer Highland
Marketing and Communications Manager
Monsenso
+45 81 75 80 01
highland@monsenso.com
Kristoffer Södersten
Director
Psykiatriresurs
+46 03 13 13 51 90
kristoffer.sodersten@psykiatriresursigoteborg.se
You can download this article as a PDF in English, Danish and Swedish

by Thomas Lethenborg | Nov 15, 2016 | News, Press releases
Copenhagen, Denmark – 15 November 2016. Monsenso announced today that the Norwegian hospital, Lovisenberg Diakonale Sykehus, will begin a pilot study with the Monsenso mHealth solution that aims to reduce hospital readmissions of individuals with bipolar disorder. Lovisenberg was signed up as a customer by Computas, Monsenso’s partner in Norway.
The pilot study, which will include individuals with bipolar disorder, aims to reduce hospital readmissions by using the Monsenso mHealth solution to intervene at an early stage.
Thomas Lethenborg, CEO at Monsenso, said that Lovisenberg would conduct a ten-month pilot study to determine if the solution should be widely implemented with other mental illnesses.
“Lovisenberg is committed to finding innovative solutions that help them provide better care to their patients in a more cost-efficient way. With the implementation of the Monsenso mHealth solution, a patient’s historical, aggregated data will be available more easily,” Mr Lethenborg added.
Kim Petersen, Executive Director at Computas AS, said “We are thrilled to have signed up our first customer with Monsenso. This type of technology gives new opportunities for the integration of mobile health into the existing mental health services in Norway; and I am confident that once clinicians start experiencing the clinical and financial benefits of implementing the solution, we will sign up more customers in no time!”
Andreas Joner, Head of Clinic at Lovisenberg, said the trial would allow them to investigate how a remote patient monitoring solution can be used to improve patient care and patient engagement.
“The Monsenso mHealth solution will help us to access and analyse data in a more efficient way. Besides, since most people carry their smartphones all the time, patients can answer their self-assessments and clinical questionnaires wherever they are, without feeling self-conscious,” said Andreas Joner.
The Monsenso mHealth solution for mental illnesses is based on a triple-loop treatment model that connects patients, carers and clinicians and has the potential to reduce hospital readmissions of bipolar patients.
Patients use a smartphone to fill in routine self-assessments that reveal their current state of mind and collect sensor data. The smartphone app also works as a self-help tool that allows patients to manage their symptoms and the behaviours that trigger those symptoms. Carers are also given a smartphone that allows them to assess the overall well-being of the one they care for, and make notes that are shared with the patient and the clinician.
Clinicians use a web portal that provides historical information on each patient, including routine self-assessments and clinical questionnaires. The portal also allows them to obtain an overview of their patients’ illness progression, symptoms, medication compliance, and medical record keeping.
With more than 1300 employees, Lovisenberg Diakonale Sykehus delivers effective treatment and outpatient care across its multiple sites. The hospital provides specialist care in mental health.
For additional information contact:
Jennifer Highland
Marketing and Communications Manager
Monsenso
+45 81 71 7713
highland@monsenso.com
Tone Hærem
Communications Director
Lovisenberg Diakonale Sykehus
tone.harem@lds.no
Pål Vermund Knudsen
Sales Director
Computas AS
pal.vermund.knudsen@computas.com
About Lovisenberg Diakonale Sykehus
Lovisenberg Diaconal Hospital provides specialised health care services in mental health and internal medicine for 180,000 inhabitants of four City Districts in Oslo (inner city). In addition, mental health services are offered to several other city boroughs. The surgical department performs scheduled operations, and has one of the leading orthopaedic units in Norway. The hospital has 234 beds, more than 2,000 employees, and an extensive out-patient facility. It is a non-profit, privately owned organisation, all services provided are within Public Health. Our mission is to offer diagnostics, treatment, and follow up services of a high quality to all our patients, with equality and respect. Being innovative and finding new solutions is part of this.
About Computas
Computas is an international IT solutions provider based in Norway, delivering solutions all over the world. We deliver services and solutions for work processes and collaboration. Our core competence is systems development, architecture and integration, project management and consulting. We have unique experience, deliver high quality in all projects, and work closely with customers to make the best solutions. The health care system in Norway is changing, and there is a need for smart solutions that can ensure quality and improve the efficiency of health services. The key to solving these challenges is through better use of IT. We are prepared to contribute with our expertise and our solutions aimed at patients and health care workers. We have delivered everything from work process solutions to apps to help the health sector to work more efficiently and to help patients to have better safety.[/vc_column_text][vc_column_text]You can download this article as PDF in English, Danish and Norwegian

by Thomas Lethenborg | Aug 30, 2016 | News, Press releases
Copenhagen, Denmark – 30 August 2016. Monsenso, the Copenhagen-based technology company developing mHealth solutions for mental illnesses, today announced a partnership with The Black Dog Institute in Australia.
Black Dog Institute is a pioneer in the identification, prevention and treatment of mental illness and the promotion of well-being. The Institute will conduct feasibility studies to determine if the Monsenso mHealth solution can be used to collect much needed data for a number of large-scale mental health trials.
“The mission of Monsenso and that of Black Dog are highly aligned. We both aim to enable better mental health care through innovation and science, therefore this is an exciting moment for both parties” said Thomas Lethenborg, CEO at Monsenso. “Monsenso is looking to build sustainable, long-term partnerships, with the ultimate goal of delivering better care, to more people, and at lower cost.”
As part of the on-going collaboration between Monsenso and the Black Dog Institute, Mads Frost, Chief Product Officer of Monsenso, has been invited to participate as a guest-speaker at the conference “Humans and Machines: A Quest for Better Mental Health” organised by Black Dog.
Helen Christensen, Director of the Black Dog Institute, said the trial would allow them to collect real-time information using a device that most people carry at all times – their Smartphone. “Our focus will be on collecting self-reported daily mood ratings and activity data that may be used to predict changes in mental health conditions over time. Ultimately these sorts of tools can be used to deliver real time assistance to those in distress at the time they most need help. However, like all good research and development projects, our aim is to test the validity of these approaches; their feasibility and the extent that they can provide better health care services. Ultimately, we hope to use these sorts of tools in our national programmes involving youth mental health and suicide prevention.”
The Monsenso mHealth solution for mental illnesses holds a CE mark and a TGA certification and it has been technically and clinically validated in clinical evaluation studies and randomised clinical trials. Furthermore, Monsenso is in the process of obtaining the ISO 13485 and ISO 27001.
For additional information contact:
Jennifer Highland
Marketing and Communications Manager
Monsenso
+45 81 71 7713
highland@monsenso.com
Gayle McNaught
Head of Public Affairs
The Black Dog Institute
g.mcnaught@blackdog.org.au
About The Black Dog Institute
The Black Dog Institute is a not-for-profit organisation which is internationally recognised as a pioneer in the identification, prevention and treatment of mental illnesses, and the promotion of well-being. We are dedicated to improving the lives of people affected by mental illness through the rapid translation of high quality research into improved clinical treatments, increased accessibility to mental health services and delivery of long-term public health solutions. Our unique approach incorporates clinical services with our cutting-edge research, our health professional training and community education programs. We combine expertise in clinical management with innovative research to develop new, and more effective, strategies for people living with mental disorders. For more information visit www.blackdoginstitute.org.au
You can download this article as PDF in English and Danish
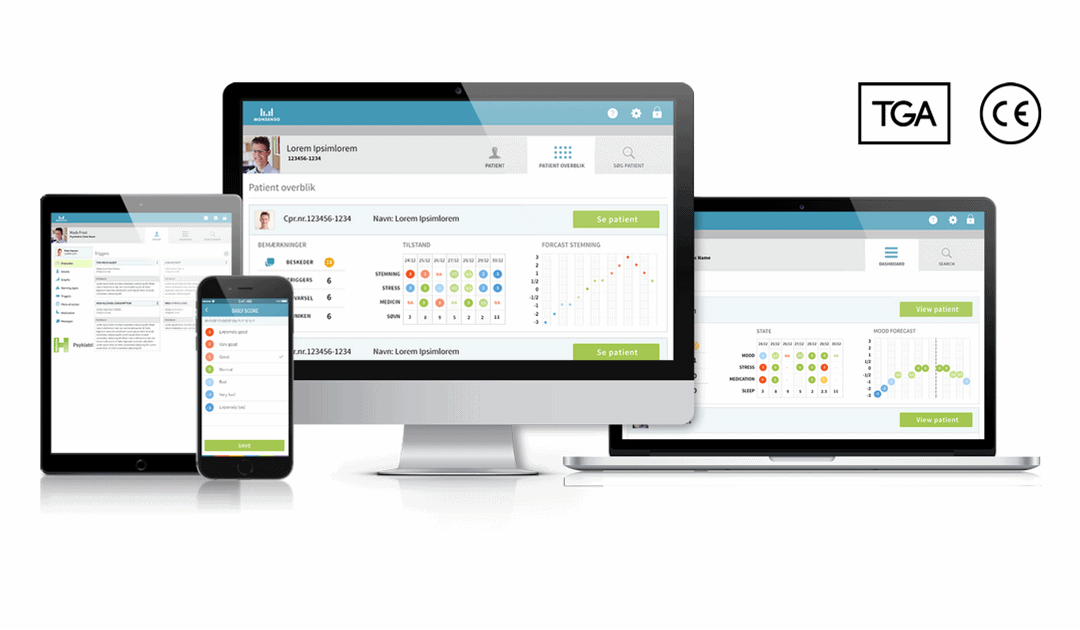
by Thomas Lethenborg | Aug 15, 2016 | News, Press releases
Copenhagen, Denmark – 16 August 2016. Monsenso, today announced that its mHealth solution received a Class I medical-device certification, from the Australian Register of Therapeutic Goods Administration (TGA).
The mHealth solution received the TGA certification in cooperation with Emergo, a consultancy company specialising in regulatory compliance for medical devices, with funding from the Danish Market Development Fund.
Thomas Lethenborg, CEO at Monsenso, commented “We are very satisfied that the Monsenso mHealth solution received the TGA certification and is now ready to be launched in the Australian market. Although we already have a customer in Australia, this certification provides us with the means to commercialise the solution nation-wide.”
This certification guarantees that a manufacturer’s product meets the essential requirements stipulated by the Australian Register of Terapeutic Administration.
“Holding the right certifications, and offering a high level of data security is essential for healthcare providers in all markets –including Australia. Therefore, it is important that Monsenso possesses the necessary quality control standards and adequate security measures to succeed world-wide” added Mr. Lethenborg.
The next steps for Monsenso are to obtain the ISO 13485 Certification; the ISO 27001 Data Security Certification; and to become HIPAA and FDA compliant. These certifications will be also obtained with the monetary support granted by the Danish Market Development Fund. You can download this article as PDF in English and Danish.
Note:
As of 23 May 2023, Monsenso does no longer hold the TGA certification.







