Contract research organisations (CRO’s)
A DIGITAL Health
solution for
DECENTRALISED Trials
Collect and analyse real-world data from clinical
trials digitally and review results effortlessly


Improve efficiency in DECENTRALISED trials
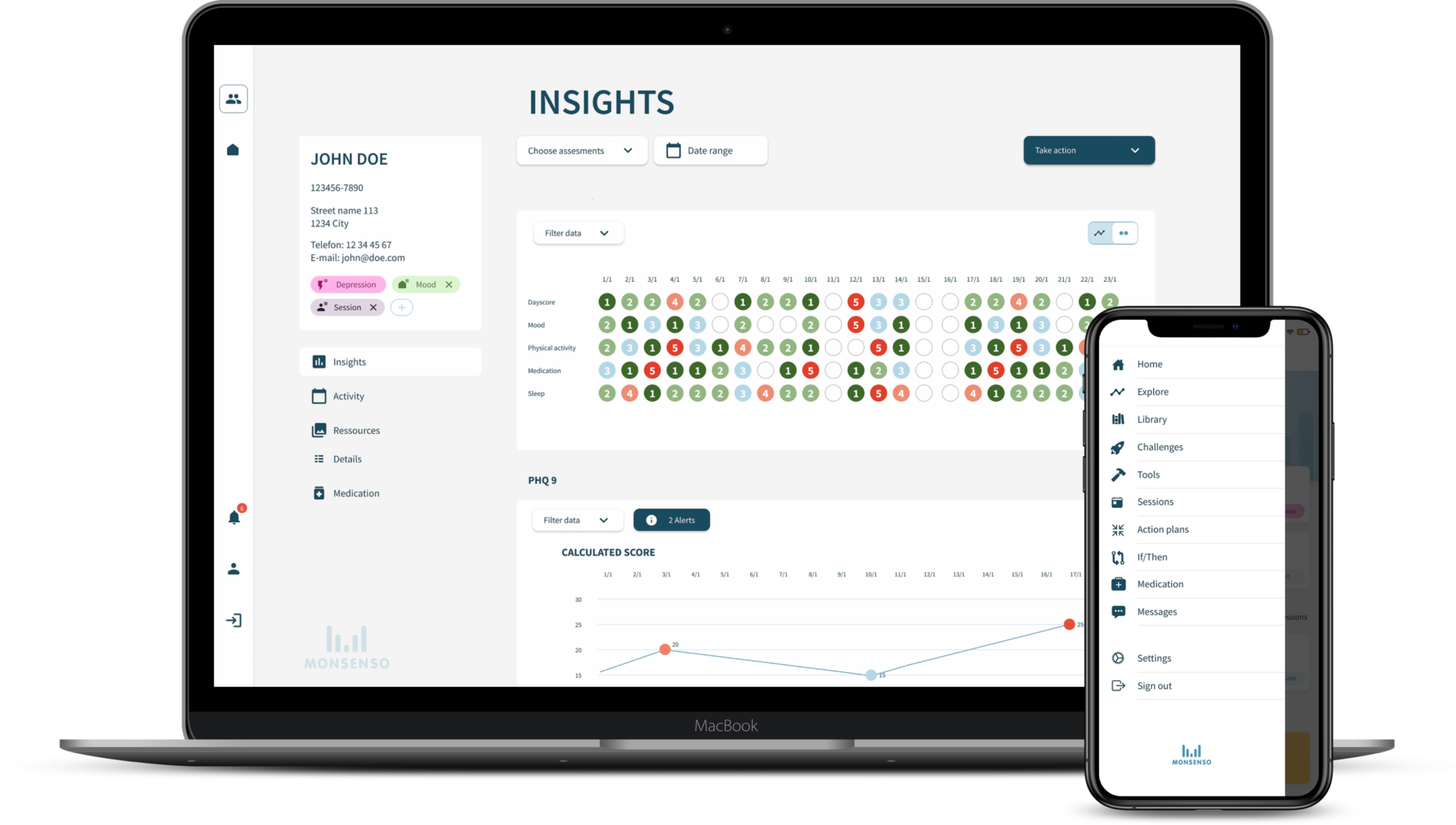
The Monsenso solution empowers CROs and pharmaceutical companies to capture real-world data, revealing insights that could enhance a trial’s efficacy and speed up the drug development process.
As our agile solution enables you to remotely assess all your subjects 24/7 during the entire length of the clinical trial, it can help increase trial participation, reach larger trial groups and reduce dropout.
Partner with us to obtain a bespoke and tailored solution.
Book a demo

Real-world data
Collect patient-reported outcomes (ePRO) i.e. self-assessments, daily diary, along with usage data, sensor data and data on voice analysis.

Convenient data analysis
The data can be viewed and analysed easily by clinicians and extracted to investigators for data analysis.

Protocol adherence
Monsenso can help driving participant engagement and protocol adherence through daily reminders.

Active trial participation
Increase trial participation and engagement by remotely monitoring your subjects.

Close the knowledge gaP
- Real-world data (RWD) can help to demonstrate real-world product-effectiveness in terms of safety and efficacy.
- RWD can help to understand broader effectiveness and acceptability amongst specific subgroups that may have not been studied in RCTs.
- RWD can help provide real-world evidence to support regulatory and payers’ requirements.
COMPLIANce, security and scalability
Monsenso adheres to the highest security standards. Our solution is a CE-marked, Class 1 Medical Device that holds the Cyber Essentials Certification. Monsenso A/S is certified in ISO 13485 and ISO 27001. and is HIPAA compliant. Read more on Monsenso’s privacy policy here.
Our solution is highly scalable for global R&D projects. It can be used by small clinics, large hospitals and health systems in 10 languages. We can configure it to your specific requirements and deliver it as a white-label product.


“Monsenso allows me to monitor patients more closely; their ups and downs. I am following them from the side-lines, and that is very reassuring for the patients”
Psychiatrist
