Pharma
DIGITAL COMPANION
FOR NEW TREATMENTS
Monsenso enables personalised care beyond the pill and
value demonstration to payers by capturing patient data
across their care journey.


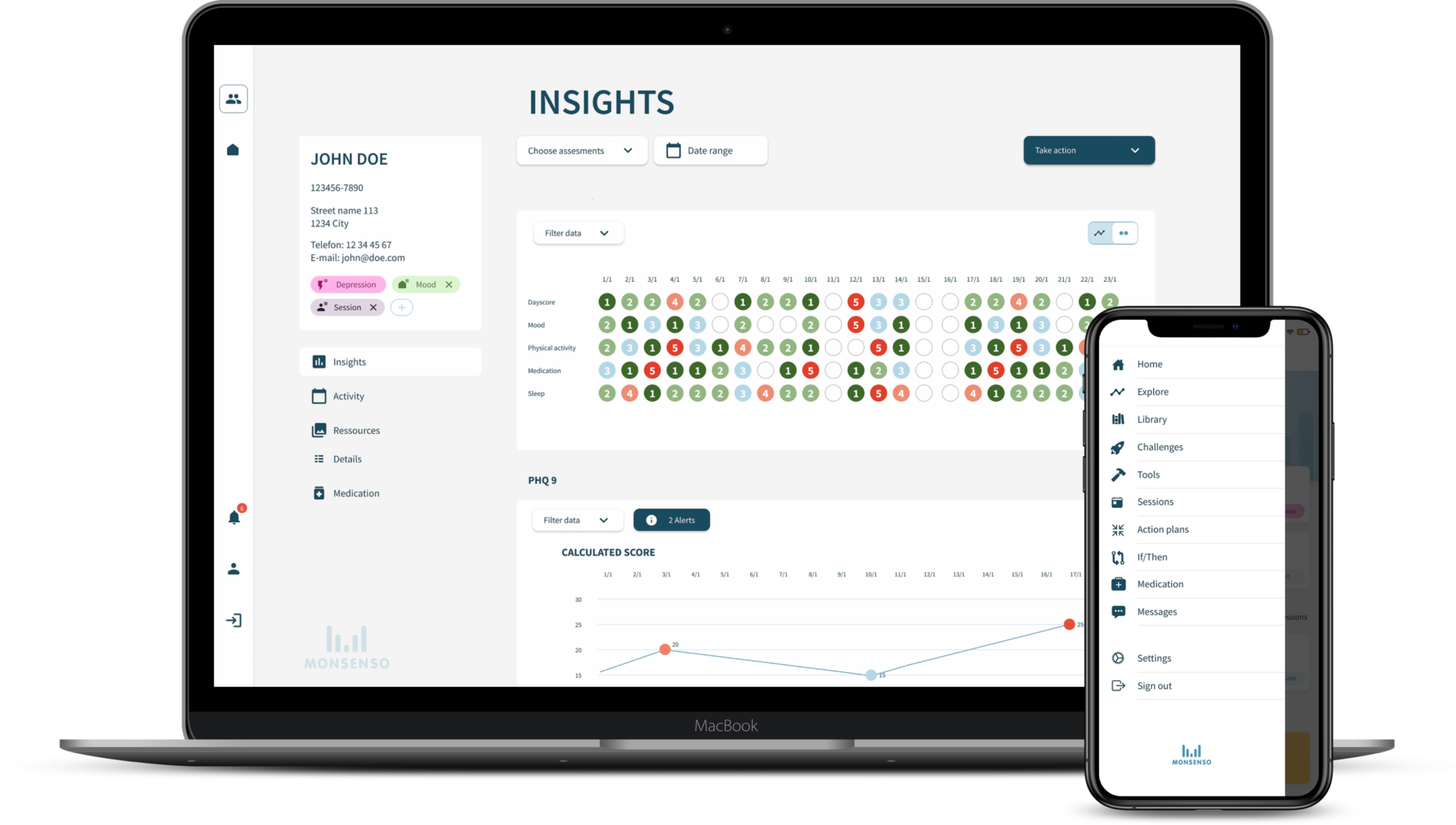
PATIENT-CENTRIC DIGITAL COMPANION
Monsenso’s digital companion solution accompanies new treatments, while complementing existing care pathways.
Our solution enables education about a new treatment, direct communication and data sharing to support the identification of treatment responders, high volume prescribers and outcomes to facilitate value-based agreements.
Our solution is a CE Class 1 medical device that captures ePRO, symptom and wearable device data to provide a holistic view of the patient journey.
VALUE DEMONSTRATIon BEYOND THE PILL
Monsenso enables personalised support and patient data capture throughout the care journey.

Patients
Collect self-reported data and provide patients with tools to practice helpful skills in day-to-day life and promote medical adherence enabling them to better manage their condition between consultations.

Clinicians
Provide clinicians with insights into patients’ symptoms, adherence and outcomes to demonstrate the value of a treatment in a transparent way.

Health systems/payers
Demonstrate real-world value to regulators and payers to support coverage, differentiate from existing treatments,
value-based reimbursement and label expansion.

IMPROVING MARKETED THERAPIES
Monsenso’s digital companion solution complements existing therapies and care pathways by empowering patients to better understand and manage their mental health between clinical visits.
Monsenso enables direct communication and data sharing between all life sciences stakeholders, enabling a personalised medication and treatment approach and improving treatment adherence.
BENEFITS OF WORKING WITH US

Therapeutic expertise
Monsenso is proud to offer expertise in a number of therapeutic areas within the mental health domain, supporting both daily clinical practice as well as clinical trials.

Track record
Extensive track record in global deployments within academia, healthcare, social care and pharmaceutical companies across a variety of mental health disorders.

Scalable solution, fast deployment
A configurable, customisable cloud-based SaaS solution, that can be quickly configured to meet the needs of the target patient population and care pathway.

Regulatory, data privacy & security
Monsenso adheres to the highest security standards. CE-marked as Class 1 Medical Device, Cyber Essentials certified and ISO 13485 and ISO 27001 compliant.
REQUEST A DEMO
Our expert team is ready to demonstrate the Monsenso digital companion solution and to answer any questions you might have.
References
[1] Lubkeman, M., Lee, Myrto., and Barrios, G. (2020) What do Patients Want, and is Pharma Delivering? Boston Consulting Group
[2] Szegedi A, Jansen WT, van Willigenburg AP, van der Meulen E, Stassen HH, Thase ME. Early improvement in the first 2 weeks as a predictor of treatment outcome in patients with major depressive disorder: a meta-analysis including 6562 patients. J Clin Psychiatry. 2009 Mar;70(3):344-53.
[3] Cohen, O., Fox, B., and Wright, P. (2020) COVID-19 and commercial pharma: Navigating and uneven recovery. McKinsey & Company
[4] Semahegn, A., Torpey, K., Manu, A. et al. (2020) Psychotropic medication non-adherence and its associated factors among patients with major psychiatric disorders: a systematic review and meta-analysis. Systems Reviews 9, 17
[5] American Medical Association: https://www.ama-assn.org/delivering-care/patient-support-advocacy/8-reasons-patients-dont-take-their-medications


“Monsenso allows me to monitor patients more closely; their ups and downs. I am following them from the side-lines, and that is very reassuring for the patients”
Psychiatrist






























