by Thomas Lethenborg | Aug 21, 2020 | News
Quality Policy and Standards
As a technology leader in mobile health (mHealth) solutions for mental health, Monsenso is ISO 13485 certified.
ISO 13485 is the gold standard for medical device companies to ensure the quality, safety and efficacy of products in the medical device field. This certification ensures that the product in question, consistently meets customer requirements and regulatory requirements applicable to medical devices and other related services.
“Monsenso adheres to the highest security standards. Beyond, being ISO 13485, Monsenso holds the ISO 27001 certifications, a TGA certification and class 1 CE mark.” says Thomas Lethenborg, CEO at Monsenso.
You can download this article as PDF in English.
For additional information contact:
Bettina van Wylich
Chief Marketing Officer
Monsenso
+45 22704724
wylich-muxoll@monsenso.com

by Thomas Lethenborg | Aug 21, 2020 | News, Press releases
Quality Policy and Standards
As a technology leader in mobile health (mHealth) solutions for mental health, Monsenso is ISO 13485 certified. ISO 13485 is the gold standard for medical device companies to ensure the quality, safety and efficacy of products in the medical device field. This certification ensures that the product in question, consistently meets customer requirements and regulatory requirements applicable to medical devices and other related services.
“Monsenso adheres to the highest security standards. Beyond, being ISO 13485 certified, Monsenso holds the ISO 27001 certification and class 1 CE mark.” says Thomas Lethenborg, CEO at Monsenso.

For additional information contact:
Jennifer Highland
Marketing and Communications Manager
Monsenso
+45 81 71 7713
highland@monsenso.com
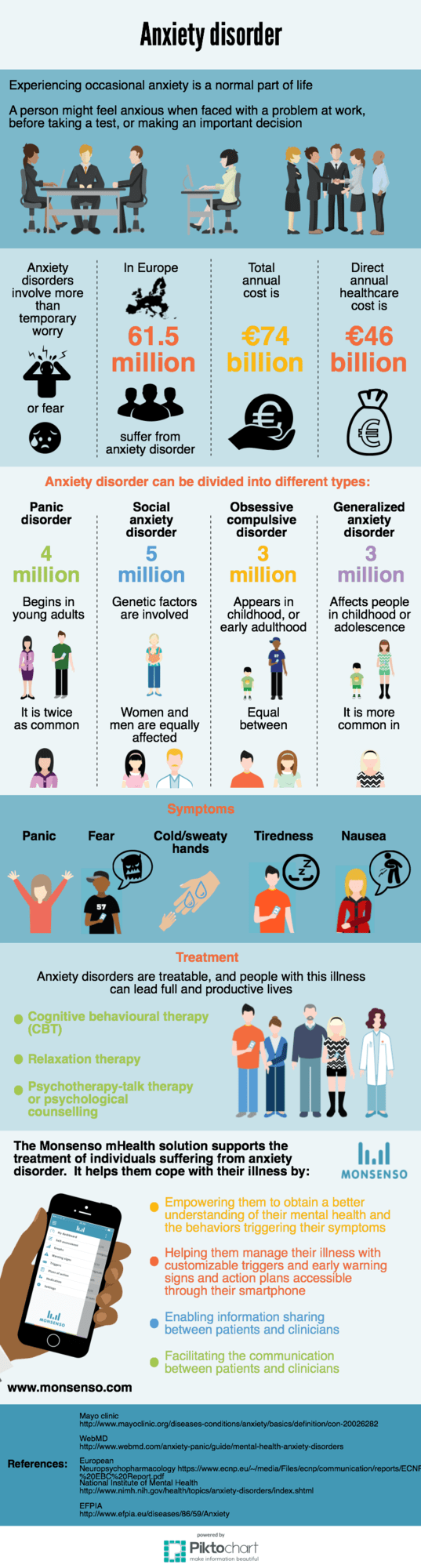
by Thomas Lethenborg | Jan 26, 2016 | Anxiety, News
Monsenso will begin clinical trials to support the treatment of anxiety disorder in collaboration with The Mental Health Services in the Region of Southern Denmark (MHS).
The Anxiety Monitoring Study
The objective of the study is to implement and validate the Monsenso mHealth solution to support the treatment of anxiety disorders.
The Monsenso mHealth platform uses a double-loop treatment model to connect care providers and individuals. Currently, it is being used to collect patients’ self-rated status and sensor-based behavioural data on a daily basis.
Recent advances in mobile technologies have created more opportunities for assessments and treatments to be available to people in situations where they are most needed. Personal health technologies can collect patients’ self-rated status and sensor-based behavioural data (e.g., physical activity, phone usage, social activity) on a daily basis.
The information gathered this way provides feedback on treatment progress to both care providers and individuals. Moreover, it also supplies ecological momentary interventions (EMI), for instance, by prompting individuals to do assignments depending on the context and patients’ current status.
Regarding anxiety disorders, mobile health solutions can be useful in assisting patients to use skills learned during treatment in real life situations, as well as to monitor and give feedback on progress or lack of progress during therapy. This feedback leads to better outcomes for patients not responding well to treatment.
Pilot study: The feasibility of the solution will be evaluated using a qualitative and quantitative study without a control group involving 30 patients for three months examining usability and usefulness of the solution for both patients and clinicians.
Participants: Anxiety Patients in Routine and Special Care Settings in Southern Denmark.
Expected results: The study will result in the development of a solution based on user needs. The platform will be a feasible, usable and useful intervention.
Implementation: If the results of the feasibility study are promising, the study results will be used to improve the mHealth solution and develop version 2. Afterwards, a randomised controlled trial (RCT) will be conducted to examine outcome related effects of the solution, such as rate of recovery. If the feasibility and outcome of the RCT studies are positive, it is expected that there will be interest in implementing the mHealth solution in routine and specialized care settings around the world.
To read the press release, click here.

by Thomas Lethenborg | Dec 3, 2015 | Bipolar, mHealth
Bipolar disorder, also known as manic-depressive illness, is a brain disorder that causes unusual shifts in mood, energy, activity levels, and the ability to carry out day-to-day tasks. [1]
People suffering from bipolar disorder will have periods or episodes of depression – where they feel very low and lethargic mania – where they feel high and overactive. [2]
Unlike simple mood swings, each episode of bipolar disorder can last for several weeks and some people may not experience a “normal” mood very often. [2]
Getting an accurate diagnosis is the first step in bipolar disorder treatment. However, this isn’t always easy. The mood swings of bipolar disorder can be difficult to distinguish from other problems such as major depression, ADHD, and borderline personality disorder. For many people suffering from bipolar disorder, it takes years and numerous doctor visits before the problem is correctly identified and treated. [3]
Indicators of bipolar disorder:
- Repeated episodes of major depression
- First episode of major depression was experienced before age 25
- First-degree relative suffering from bipolar disorder
- Mood and energy levels are higher than most people’s when not depressed
- Oversleeping and overeating when depressed
- Episodes of major depression are shorter than 3 months
- Lost contact with reality while depressed
- Suffered from postpartum depression in the past
- Developed mania or hypomania while taking antidepressants
- Antidepressants stopped working after several months
- Tried three or more antidepressants without success [3]
If a person is not treated, episodes of bipolar-related mania can last for between three to six months. Episodes of depression tend to last longer, for between six and twelve months. However, with effective treatment, episodes usually improve within about three months. [2]
Most people with bipolar disorder can be treated using a combination of different treatments that can include:
- Medication such as mood stabilisers and antidepressants
- Learning to recognize triggers and early warning signs of an episode of depression or mania
- Psychotherapy to deal with depression and provide advice on how to improve relationships
- Lifestyle advice such as doing regular exercise, planning activities you enjoy that give you a sense of achievement, and advice on improving your diet and getting more sleep [2]
Mobile health technology
The Monsenso mHealth platform is based on The MONARCA Research Project, aimed at developing and validating a solution for multi-parametric, long-term monitoring of behavioral and physiological information relevant to bipolar disorder.
The Monsenso solution can help predict and prevent episodes by training patients to recognize their early warning signs, which are symptoms that indicate an oncoming episode [4].
In particular, during the research project, it was discovered that these three parameters are crucial in keeping a bipolar patient stable:
- Adherence to prescribed medication: Taking all medications on a daily basis, exactly as prescribed.
- Stable sleep patterns: Sleeping eight hours every night and maintaining a consistent routine of going to bed, waking up.
- Staying active both physically and socially: Getting out of the house every day, going to work, and engaging in social interaction.
Therefore, the Monsenso solution includes five core features that support a patient’s self-management:
- Self-assessments – Reminded by an alarm, patients enter subjective data directly into the system through their smartphones. This data includes mood, sleep, level of activity, and medication. Some items can be customized to accommodate a patient’s specific needs, while others are consistent to provide statistical analysis.
- Activity monitoring – Through a GPS and accelerometer, objective data is collected to monitor a patient’s level of engagement in daily activities. The system can also measure the amount of social activity based on phone calls and text messages.
- Historical overview of data – On the web portal, patients and clinicians can obtain a two-week snapshot of a patient’s basic data for immediate feedback. The portal also gives them access to a detailed historical overview of the data, enabling them to explore it in depth by going back in time, and focusing on specific variables.
- Coaching and self-treatment – The MONARCA systems supported psychotherapy in two ways. Firstly, through customizable triggers that notify the patient and clinician when the data potentially indicates a warning sign. Second, since the patients have access to their own Early Warning Signs, it empowers them to learn more about them.
- Data sharing – To strengthen the relationship between patients and clinicians, important information and treatment decisions are shared.
Resources:
[1] What is bipolar disorder? National Institute of Mental Health. http://www.nimh.nih.gov/health/topics/bipolar-disorder/index.shtml
[2] Bipolar disorder. National Health Service (NHS) UK. http://www.nhs.uk/Conditions/Bipolar-disorder/Pages/Introduction.aspx
[3] Bipolar disorder treatment. HelpGuide.org http://www.helpguide.org/articles/bipolar-disorder/bipolar-disorder-treatment.htm